Research and Development
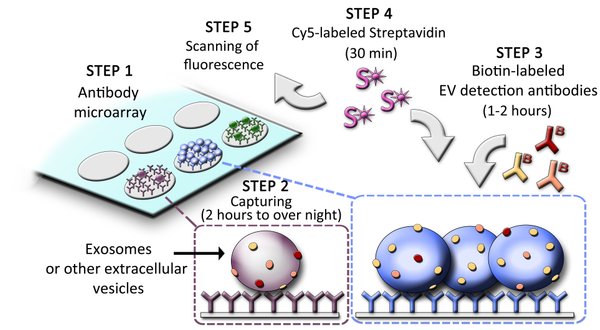
Principle of the Extracelluar Vesicle Array
EV Array
Step 1: The EV Array is composed of different capture antibodies printed on a microarray slide.
Step 2: 10-100 µL plasma or other body fluids (urine, saliva, BALF, etc.) are applied in a 96-well setup and incubated 2 hours to overnight.
Step 3: The EVs are detected with a cocktail of biotinylated antibodies.
Step 4/5: The presence and thereby phenotype of EVs is visualized after incubation with Cy5-labeled Streptavidin using a fluorescence scanner.
The use of microarray as a platform with spots of 1 nL volumes minimizes the cost of the EV Array as only small amounts of antibodies are needed.
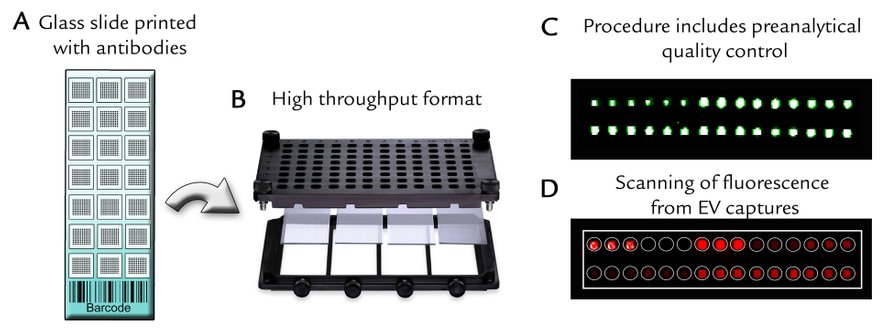
The EV Array in Practice
A) Barcoded microarray glass slides (7.5 x 2.5cm) printed in a 21-well setup.
B) Printed slides are placed in multiwell-cassettes for either one glass slide (up to 21 samples) or four glass slides (up to 84 samples).
C) The procedure is validated by a quality control of the printed spots, which includes positive and negative controls.
D) Scanning after capture and detection of EVs can be performed on a normal laboratory fluorescence scanner or on a specialized microarray scanner for maximum sensitivity.
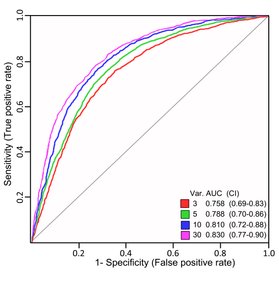
The Diagnostic and Prognostic Power of the EV Array
The diagnostic power of the EVs to identify LUNG CANCER patients can be visualized by the receiver operating characteristics (ROC), which shows the models’ sensitivity, specificity and accuracy.
The data presented was obtained by analyzing EVs from 10 µL of plasma from 109 lung cancer patients (NSCLC) together with 110 lung-diseased patients (non-cancerous). Thirty-seven different EV markers were analyzed simultaneously and a multivariate data analysis was performed.
Using the results from 30 of the variables it was possible to distinguish lung cancer patients from other lung diseased patients with a SPECIFICITY OF 0.79 and a SENSITIVITY OF 0.73 with an ACCURACY OF 76% (Jakobsen et al., 2015).